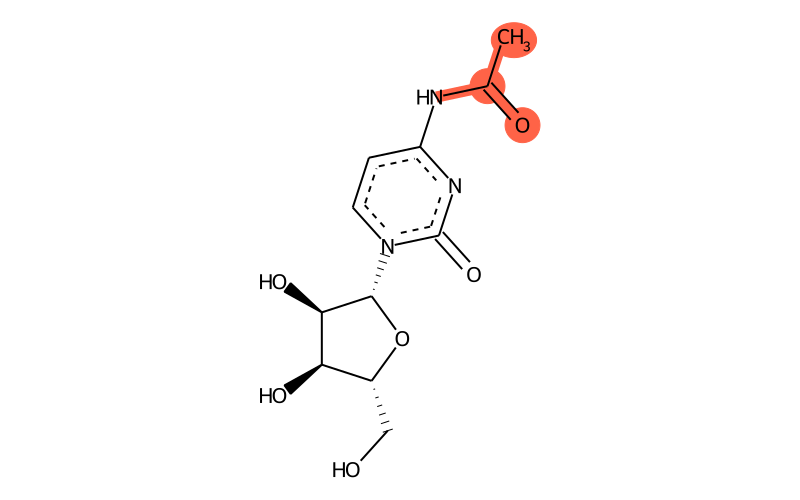
Summary
| Full name | N4-acetylcytidine |
| IUPAC name | N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]acetamide |
| Short name | ac4C |
| MODOMICS code new | 2000000042C |
| MODOMICS code | 42C |
| Synonyms |
3597-45-3
3768-18-1 4-[(1-Hydroxyethylidene)amino]-1-pentofuranosylpyrimidin-2(1H)-one 4-Acetyl-1-(beta-delta-ribofuranosyl)cytosine 4-Acetyl-1-(beta-D-ribofuranosyl)cytosine 4-Acetylcytidine 4-N-acetyl-cytidine 768A181 AC1L326V AC1Q5JLE AC-32170 Acetamide, N-(1,2-dihydro-2-oxo-1-beta-D-ribofuranosyl-4-pyrimidinyl) Acetamide, N-(1,2-dihydro-2-oxo-1-.beta.-D-ribofuranosyl-4-pyrimidinyl)- acetyl cytosine Acetylcytidine AJ-50600 AKOS015896940 AN-20958 AR-1K5740 AS-12329 C11-H15-N3-O6 C11H15N3O6 C22293 CA-237 CHEBI:70989 CID107461 CJ-11922 CS-0030784 CTK1C3669 Cytidine, N-acetyl- DTXSID40958718 EINECS 223-195-6 HY-W019670 K-8298 MFCD00006540 N-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-yl}acetamide N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]acetamide N-(1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)acetamide N-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-2-oxo-1,2-dihydropyrimidin-4-yl}acetamide N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-2-oxo-pyrimidin-4-yl]acetamide N4 -Acetylcytidine n4-acetyl cytidine N-4-Acetylcytidine N4-Acetylcytidine n(4)-acetylcytidine N4-Acetylcytidine, 97.5% - 1G 1g N4-Acetylcytidine, >=98% n4-Acetylcytidine;n-acetylcytidine n-acetyl-Cytidine N-Acetylcytidine NIDVTARKFBZMOT-PEBGCTIMSA-N NS00014881 Q20890503 RT-001601 SC-09055 SCHEMBL453447 ST2419678 TL8002770 ZINC4321512 |
| Nature of the modified residue | Natural |
| RNAMods code | M |
| Residue unique ID | 81 |
| Found in RNA | Yes |
| Related nucleotides | 200 |
| Enzymes |
MTH909 (Methanothermobacter thermautotrophicus) NAT10 (Homo sapiens) Rra1 (Saccharomyces cerevisiae) TmcA (Escherichia coli) TmcA (Haloferax volcanii) |
| Found in phylogeny | Eubacteria, Eukaryota |
| Found naturally in RNA types | rRNA, tRNA |
Chemical information
* Chemical properties calculated with Open Babel - O'Boyle et al. Open Babel: An open chemical toolbox. J Cheminform 3, 33 (2011) (link)
QM Data:
| Dipole Magnitude [D]: | 3.520579316 |
| Energy [Eh]: | -1043.45069726262 |
| HOMO [eV]: | -9.3479 |
| LUMO [eV]: | 0.2129 |
| Gap [eV]: | 9.5608 |
Download QM Data:
| Charges | charge.txt |
Download Structures
| 2D | .png .mol .mol2 .sdf .pdb .smi |
| 3D | .mol .mol2 .sdf .pdb |
Tautomers
| Tautomers SMILES |
CC(N=c1[nH]c(=O)n(C2C(O)C(O)C(CO)O2)cc1)=O tautomer #0
CC(Nc1nc(=O)n(C2C(O)C(O)C(CO)O2)cc1)=O tautomer #1 C=C(N=c1[nH]c(=O)n(C2C(O)C(O)C(CO)O2)cc1)O tautomer #2 C=C(Nc1nc(=O)n(C2C(O)C(O)C(CO)O2)cc1)O tautomer #3 CC(N=c1nc(O)n(C2C(O)C(O)C(CO)O2)cc1)=O tautomer #4 CC(=Nc1nc(=O)n(C2C(O)C(O)C(CO)O2)cc1)O tautomer #5 C=C(N=c1nc(O)n(C2C(O)C(O)C(CO)O2)cc1)O tautomer #6 |
| Tautomer image | Show Image |
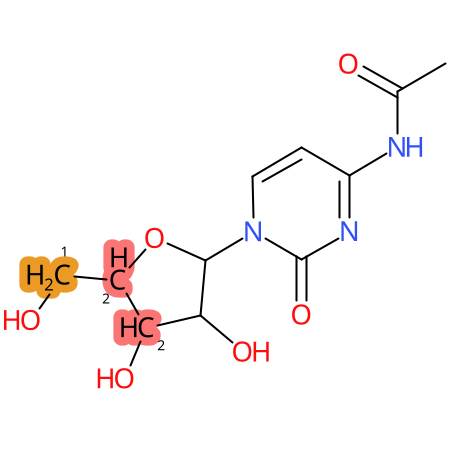
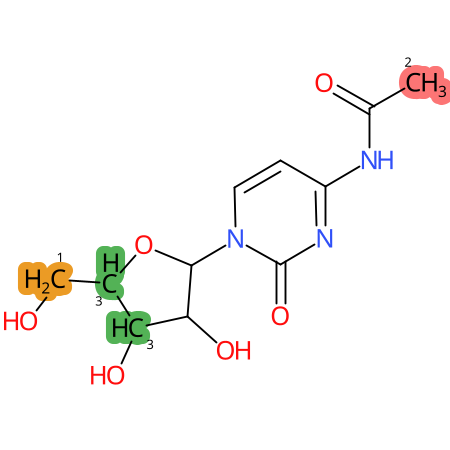
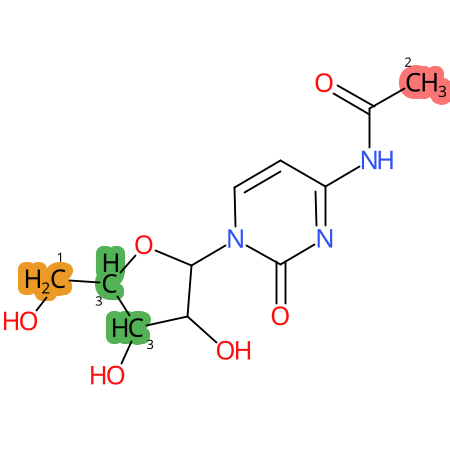
Predicted CYP Metabolic Sites
| CYP3A4 | CYP2D6 | CYP2C9 |
|---|---|---|
|
|
|
* CYP Metabolic sites predicted with SMARTCyp. SMARTCyp is a method for prediction of which sites in a molecule that are most liable to metabolism by Cytochrome P450. It has been shown to be applicable to metabolism by the isoforms 1A2, 2A6, 2B6, 2C8, 2C19, 2E1, and 3A4 (CYP3A4), and specific models for the isoform 2C9 (CYP2C9) and isoform 2D6 (CYP2D6). CYP3A4, CYP2D6, and CYP2C9 are the three of the most important enzymes in drug metabolism since they are involved in the metabolism of more than half of the drugs used today. The three top-ranked atoms are highlighted. See: SmartCYP and SmartCYP - background; Patrik Rydberg, David E. Gloriam, Lars Olsen, The SMARTCyp cytochrome P450 metabolism prediction server, Bioinformatics, Volume 26, Issue 23, 1 December 2010, Pages 2988–2989 (link)
LC-MS Information
| Monoisotopic mass | 285.0961 |
| Average mass | 285.253 |
| [M+H]+ | 286.1039 |
| Product ions | 154/112 |
| Normalized LC elution time * | 1,35 (Kellner 2014) |
| LC elution order/characteristics | between G and A (Kellner 2014) |
* normalized to guanosine (G), measured with a RP C-18 column with acetonitrile/ammonium acetate as mobile phase.
LC-MS Publications
| Title | Authors | Journal | Details | ||
|---|---|---|---|---|---|
| Profiling of RNA modifications by multiplexed stable isotope labelling. | Kellner S, Neumann J, Rosenkranz D, Lebedeva S, Ketting RF, Zischler H, Schneider D, Helm M. | Chem Commun (Camb). | [details] | 24567952 | - |
| Absolute and relative quantification of RNA modifications via biosynthetic isotopomers. | Kellner S, Ochel A, Thuring K, Spenkuch F, Neumann J, Sharma S, Entian KD, Schneider D, Helm M... | Nucleic Acids Res | [details] | 25129236 | - |
Chemical groups contained
| Type | Subtype |
|---|---|
| other | acetylamide |
Reactions producing N4-acetylcytidine
| Name |
|---|
| C:ac4C |
Reactions starting from N4-acetylcytidine
| Name |
|---|
| ac4C:ac4Cm |
Last modification of this entry: Sept. 15, 2025