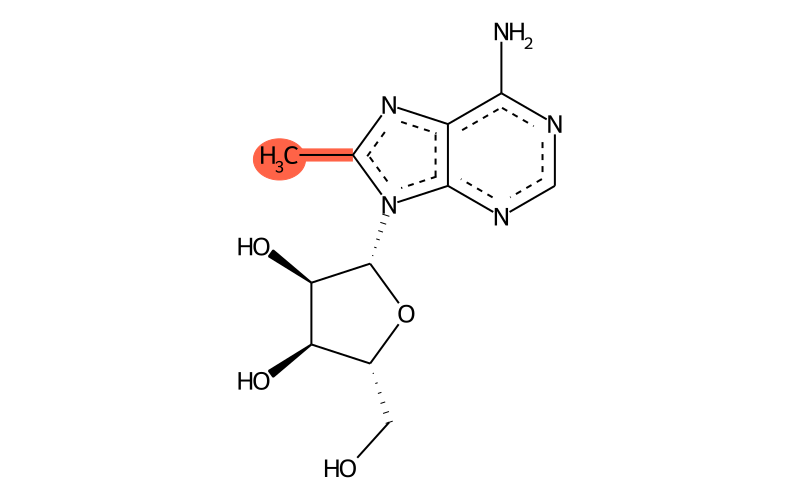
Summary
| Full name | 8-methyladenosine |
| IUPAC name | (2R,3R,4S,5R)-2-(6-amino-8-methylpurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |
| Short name | m8A |
| MODOMICS code new | 2000000008A |
| MODOMICS code | 8A |
| Synonyms |
2-(6-Amino-8-methyl-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol
(2R,3R,4S,5R)-2-(6-Amino-8-methyl-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (2R,3R,4S,5R)-2-(6-amino-8-methylpurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol (2R,3R,4S,5R)-2-(6-amino-8-methyl-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol 56973-12-7 8-Methyl adenosine 8-Methyl Ado 8-Methyladenosine AC1L57Q5 Adenosine, 8-methyl- Adenosine, 8-methyl-(9CI) CID171555 CTK5A6087 DTXSID40205572 RTGYRFMTJZYXPD-IOSLPCCCSA-N SCHEMBL967122 ZINC5161492 |
| Nature of the modified residue | Natural |
| RNAMods code | â |
| Residue unique ID | 131 |
| Found in RNA | Yes |
| Related nucleotides | 410 |
| Enzymes |
Cfr (Staphylococcus sciuri) |
| Found in phylogeny | Eubacteria |
| Found naturally in RNA types | rRNA |
Chemical information
| Sum formula | C11H15N5O4 |
| Type of moiety | nucleoside |
| Degeneracy | not applicable |
| SMILES | Cc1nc2c(N)ncnc2[n]1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |
| logP | -1.0904 |
| TPSA | 139.54 |
| Number of atoms | 20 |
| Number of Hydrogen Bond Acceptors 1 (HBA1) | 8 |
| Number of Hydrogen Bond Acceptors 2 (HBA2) | 9 |
| Number of Hydrogen Bond Donors (HBD) | 4 |
| InChI | InChI=1S/C11H15N5O4/c1-4-15-6-9(12)13-3-14-10(6)16(4)11-8(19)7(18)5(2-17)20-11/h3,5,7-8,11,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 |
| InChIKey | RTGYRFMTJZYXPD-IOSLPCCCSA-N |
| Search the molecule in external databases | ChEMBL PubChem Compound Database Ligand Expo WIPO |
| PubChem CID | |
| PubChem SIDs |
* Chemical properties calculated with Open Babel - O'Boyle et al. Open Babel: An open chemical toolbox. J Cheminform 3, 33 (2011) (link)
QM Data:
| Dipole Magnitude [D]: | 5.047563813 |
| Energy [Eh]: | -1002.53383804998 |
| HOMO [eV]: | -8.7001 |
| LUMO [eV]: | 1.163 |
| Gap [eV]: | 9.8631 |
Download QM Data:
| Charges | charge.txt |
Download Structures
| 2D | .png .mol .mol2 .sdf .pdb .smi |
| 3D | .mol .mol2 .sdf .pdb |
Tautomers
| Tautomers SMILES |
Cc1nc2c(N)ncnc2n1C3OC(CO)C(O)C3O tautomer #0
Cc1nc2c(N)ncnc2n1C3OC(CO)C(O)C3O tautomer #1 C=C1Nc2c(N)ncnc2N1C3OC(CO)C(O)C3O tautomer #2 C=C1Nc2c(N)ncnc2N1C3OC(CO)C(O)C3O tautomer #3 Cc1nc2c(=N)nc[nH]c2n1C3OC(CO)C(O)C3O tautomer #4 Cc1nc2c(=N)[nH]cnc2n1C3OC(CO)C(O)C3O tautomer #5 CC1=NC2C(=N)N=CN=C2N1C3OC(CO)C(O)C3O tautomer #6 C=c1nc2C(N)N=CN=c2n1C3OC(CO)C(O)C3O tautomer #7 C=C1N=C2C(N)=NC=NC2N1C3OC(CO)C(O)C3O tautomer #8 C=C1NC2C(=N)N=CN=C2N1C3OC(CO)C(O)C3O tautomer #9 C=C1N=C2C(=N)N=CNC2N1C3OC(CO)C(O)C3O tautomer #10 C=C1N=C2C(=N)NC=NC2N1C3OC(CO)C(O)C3O tautomer #11 C=C1Nc2c(=N)nc[nH]c2N1C3OC(CO)C(O)C3O tautomer #12 C=C1Nc2c(=N)[nH]cnc2N1C3OC(CO)C(O)C3O tautomer #13 |
| Tautomer image | Show Image |
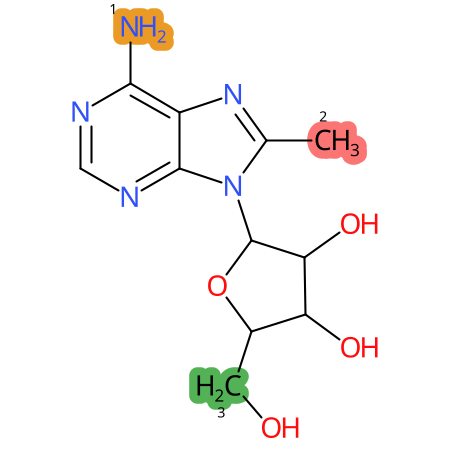
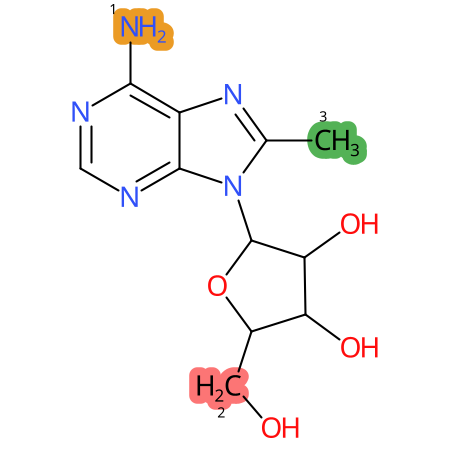
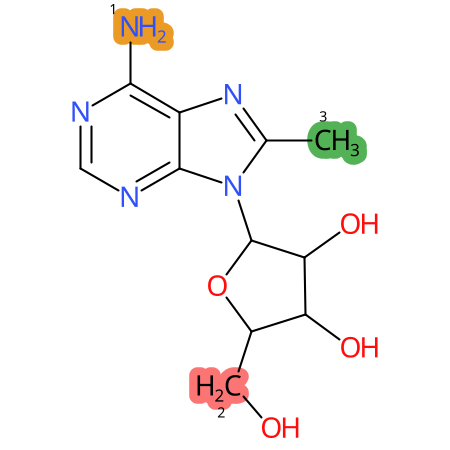
Predicted CYP Metabolic Sites
| CYP3A4 | CYP2D6 | CYP2C9 |
|---|---|---|
|
|
|
* CYP Metabolic sites predicted with SMARTCyp. SMARTCyp is a method for prediction of which sites in a molecule that are most liable to metabolism by Cytochrome P450. It has been shown to be applicable to metabolism by the isoforms 1A2, 2A6, 2B6, 2C8, 2C19, 2E1, and 3A4 (CYP3A4), and specific models for the isoform 2C9 (CYP2C9) and isoform 2D6 (CYP2D6). CYP3A4, CYP2D6, and CYP2C9 are the three of the most important enzymes in drug metabolism since they are involved in the metabolism of more than half of the drugs used today. The three top-ranked atoms are highlighted. See: SmartCYP and SmartCYP - background; Patrik Rydberg, David E. Gloriam, Lars Olsen, The SMARTCyp cytochrome P450 metabolism prediction server, Bioinformatics, Volume 26, Issue 23, 1 December 2010, Pages 2988–2989 (link)
LC-MS Information
| Monoisotopic mass | 281.1124 |
| Average mass | 281.268 |
| [M+H]+ | 282.1202 |
| Product ions | 150 |
| Normalized LC elution time * | not available |
| LC elution order/characteristics | not available |
* normalized to guanosine (G), measured with a RP C-18 column with acetonitrile/ammonium acetate as mobile phase.
Comments
The methyltransferase Cfr making m8A, has only been found in Staphylococcus sciuri so far, but it has been investigated in E. coli by expression of Cfr from a plasmid coded gene. It methylates A2503 in E. coli 23S RNA to m8A.
Also see:
Identification of 8-methyladenosine as the modification catalyzed by the
radical SAM methyltransferase Cfr that confers antibiotic resistance in
bacteria. Giessing AM, Jensen SS, Rasmussen A, Hansen LH, Gondela A, Long K, Vester B,
Kirpekar F. RNA. 2009 Feb;15(2):327-36.
PMID: 19144912
Chemical groups contained
| Type | Subtype |
|---|---|
| methyl group | methyl at aromatic C |
Reactions producing 8-methyladenosine
| Name |
|---|
| A:m8A |
Reactions starting from 8-methyladenosine
| Name |
|---|
| m8A:m2,8A |
Last modification of this entry: Sept. 15, 2025