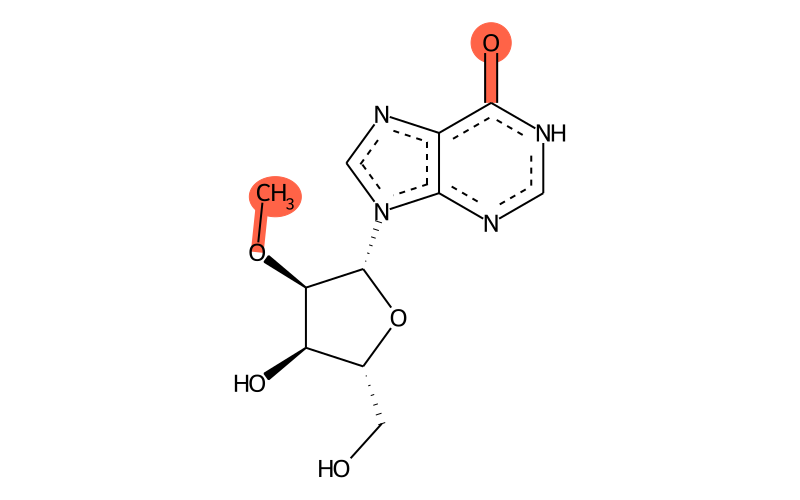
Summary
| Full name | 2'-O-methylinosine |
| IUPAC name | 9-[(2R,3R,4R,5R)-4-hydroxy-5-(hydroxymethyl)-3-methoxyoxolan-2-yl]-1H-purin-6-one |
| Short name | Im |
| MODOMICS code new | 2000000909A |
| MODOMICS code | 09A |
| Synonyms |
2-Chloro-6-fluorobenzotrichloride
2'-O-METHYL-D-INOSINE 2?O-Methylinosine 2'-(o-Methyl)-inosine 2'-O-methylinosine 2'-O-Methyl-inosine 2-(O-METHYL)-INOSINE 3881-21-8 3'-O-Methyl-Inosine 881M218 9-[(2R,3R,4R,5R)-4-HYDROXY-5-(HYDROXYMETHYL)-3-METHOXYOXOLAN-2-YL]-1H-PURIN-6-ONE 9-((2R,3R,4R,5R)-4-hydroxy-5-(hydroxymethyl)-3-methoxytetrahydrofuran-2-yl)-1H-purin-6(9H)-one 9-[(2R,3R,4R,5R)-4-hydroxy-5-(hydroxymethyl)-3-methoxy-tetrahydrofuran-2-yl]-3H-purin-6-one 9-((2R,3R,4R,5R)-4-hydroxy-5-(hydroxymethyl)-3-methoxytetrahydrofuran-2-yl)-9H-purin-6-ol 9-[(2R,3R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-3-methoxy-oxolan-2-yl]-3H-purin-6-one AC-8215 AKOS025402340 AN-8215 CHEBI:68467 CID10062287 CTK8F4496 DTXSID00435030 HPHXOIULGYVAKW-IOSLPCCCSA-N J-700080 MFCD01631008 NS00014739 Q15632692 SCHEMBL658713 VZ21766 ZINC34149906 |
| Nature of the modified residue | Natural |
| RNAMods code | Ш |
| Residue unique ID | 128 |
| Found in RNA | Yes |
| Related nucleotides | 367 |
Chemical information
| Sum formula | C11H14N4O5 |
| Type of moiety | nucleoside |
| Degeneracy | not applicable |
| ChEBI ID | 68467 |
| Reaxys Registry Number | 562424 |
| SMILES | CO[C@H]1[C@H]([n]2cnc3c2nc[nH]c3=O)O[C@H](CO)[C@H]1O |
| logP | -1.6148 |
| TPSA | 122.49 |
| Number of atoms | 20 |
| Number of Hydrogen Bond Acceptors 1 (HBA1) | 7 |
| Number of Hydrogen Bond Acceptors 2 (HBA2) | 8 |
| Number of Hydrogen Bond Donors (HBD) | 3 |
| InChI | InChI=1S/C11H14N4O5/c1-19-8-7(17)5(2-16)20-11(8)15-4-14-6-9(15)12-3-13-10(6)18/h3-5,7-8,11,16-17H,2H2,1H3,(H,12,13,18)/t5-,7-,8-,11-/m1/s1 |
| InChIKey | HPHXOIULGYVAKW-IOSLPCCCSA-N |
| Search the molecule in external databases | ChEMBL PubChem Compound Database Ligand Expo WIPO |
| PubChem CID | |
| PubChem SIDs |
15046798
44179318 79570041 126617342 126664468 128093780 160645712 163129407 206253751 222493929 223669264 225075322 226956609 252225444 260399922 274825940 304738027 310276821 310917173 312244632 312343725 315373926 315965342 316538429 316900028 322091962 341124652 341321993 341844340 342465016 347661073 349025873 363671141 375118961 375540692 377991419 381990237 385011995 385533581 385644302 386480618 387100400 389340052 419542663 438549127 441092157 442862468 443520498 |
* Chemical properties calculated with Open Babel - O'Boyle et al. Open Babel: An open chemical toolbox. J Cheminform 3, 33 (2011) (link)
QM Data:
| Dipole Magnitude [D]: | 7.40182735 |
| Energy [Eh]: | -1022.35996443254 |
| HOMO [eV]: | -9.1017 |
| LUMO [eV]: | 0.9935 |
| Gap [eV]: | 10.0952 |
Download QM Data:
| Charges | charge.txt |
Download Structures
| 2D | .png .mol .mol2 .sdf .pdb .smi |
| 3D | .mol .mol2 .sdf .pdb |
Tautomers
| Tautomers SMILES |
COC1C(n2cnc3c2ncnc3O)OC(CO)C1O tautomer #0
COC1C(n2cnc3c2[nH]cnc3=O)OC(CO)C1O tautomer #1 COC1C(n2cnc3c2nc[nH]c3=O)OC(CO)C1O tautomer #2 COC1C(n2cnc3c2ncnc3O)OC(CO)C1O tautomer #3 COC1C(N2C=NC3C2=NC=NC3=O)OC(CO)C1O tautomer #4 |
| Tautomer image | Show Image |
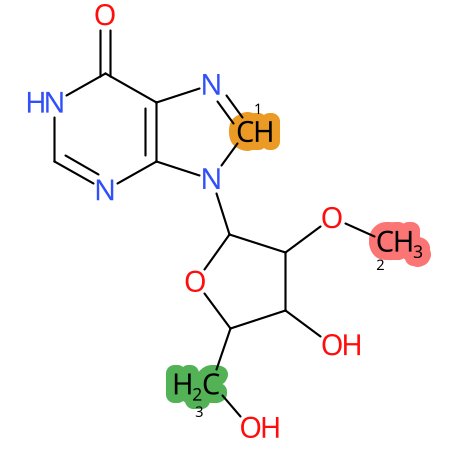
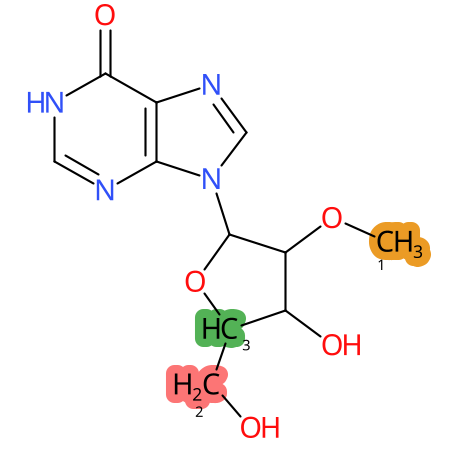
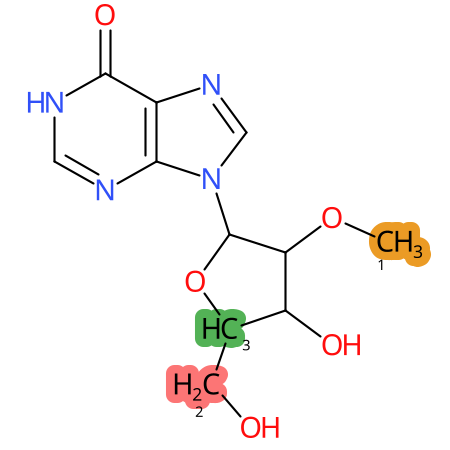
Predicted CYP Metabolic Sites
| CYP3A4 | CYP2D6 | CYP2C9 |
|---|---|---|
|
|
|
* CYP Metabolic sites predicted with SMARTCyp. SMARTCyp is a method for prediction of which sites in a molecule that are most liable to metabolism by Cytochrome P450. It has been shown to be applicable to metabolism by the isoforms 1A2, 2A6, 2B6, 2C8, 2C19, 2E1, and 3A4 (CYP3A4), and specific models for the isoform 2C9 (CYP2C9) and isoform 2D6 (CYP2D6). CYP3A4, CYP2D6, and CYP2C9 are the three of the most important enzymes in drug metabolism since they are involved in the metabolism of more than half of the drugs used today. The three top-ranked atoms are highlighted. See: SmartCYP and SmartCYP - background; Patrik Rydberg, David E. Gloriam, Lars Olsen, The SMARTCyp cytochrome P450 metabolism prediction server, Bioinformatics, Volume 26, Issue 23, 1 December 2010, Pages 2988–2989 (link)
LC-MS Information
| Monoisotopic mass | 282.0964 |
| Average mass | 282.253 |
| [M+H]+ | 283.1042 |
| Product ions | 137 |
| Normalized LC elution time * | not available |
| LC elution order/characteristics | not available |
* normalized to guanosine (G), measured with a RP C-18 column with acetonitrile/ammonium acetate as mobile phase.
Chemical groups contained
| Type | Subtype |
|---|---|
| methyl group | methyl at O2 |
| other | keto |
Reactions producing 2'-O-methylinosine
| Name |
|---|
| I:Im |
| Am:Im |
Reactions starting from 2'-O-methylinosine
| Name |
|---|
| Im:m1Im |
Last modification of this entry: Sept. 15, 2025